http://www.iop.org/EJ/abstract/0960-1317/17/5/017/
by:
In this study, ink-jet printing techniques were used to directly deposit metallic conductive patterns to produce wiring boards, antennas, electrodes and so forth. In these methods, aqueous solutions of metal salt and reducing agent were ink-jet printed consecutively onto the substrate, where an immediate chemical reduction transformed the metal cations into very fine metallic particles. The best performing reducing agent for ink-jet metal deposition was found to be ascorbic acid at neutral pH. Using this chemistry, nanosized silver patterns, composed of particles in the size range 10–200 nm, were successfully formed using a standard office ink-jet thermal-head printer. Deposited layers of silver with high electrical conductance up to 1.89 × 105 S m-1 and sheet resistance up to 0.5 Ohms/m were printed whilst higher conductivities might be expected using more appropriate devices.
...
Chemicals
Sodium and zinc formaldehyde sulfoxylate were obtained from BASF along with Cyclanon ECO, all as commercial grade materials. Chemicals such as ascorbic acid (83.3%), hydroxylamine, sodium borohydride, copper salts, i.e. copper sulfate, copper nitrate, etc, and silver salts, i.e. silver nitrate, carbonate, acetate, chloride, etc,were purchased from different chemical suppliers (Avocado, Lancaster, Fluka and Aldrich). Copper sulfate and silver nitrate, as the main metallic salts used in the experiments, were laboratory grade and all other chemicals were selected as analytical grade. Different substrates were used in the final ink-jet metal deposition tests including A4 size copying paper, A4 size inkjet transparency film and woven fabrics made up of 100% cotton. A4 size copying paper of 80 g m-2 (80 GSM) and transparency films (polyester film with hydrophilic coating) were purchased from Guilbert. 100% bleached cotton fabric (plain weaved, 190 gm-2) was obtained locally (from Whalley, Bradford).
Ink-jet deposition plan
For printing purposes a Hewlett Packard (HP) single head office ink-jet printer (Apollo 1200) was used to print metallic salt and/or reducing agent solutions in separate runs. Usually the substrate was first printed with the reducing ink and then with metal precursor ink. Microsoft Word was used as the printer controlling software. The substrate could be an A4 size sheet of paper, an ink-jet transparency film or a piece of fabric glued to paper.
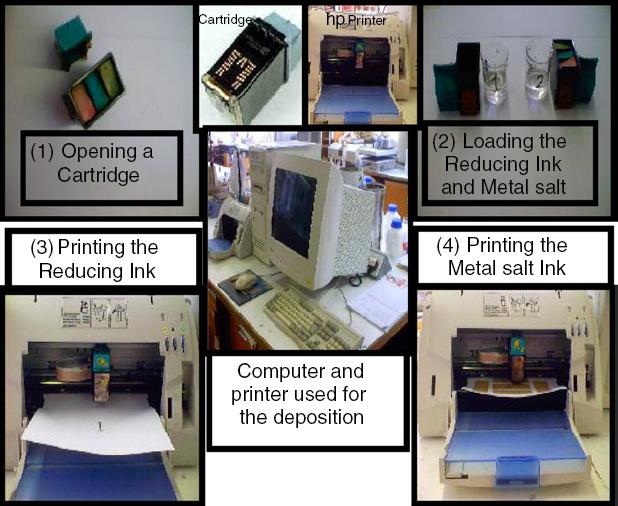
A full deposition cycle in a two-ink (metal salt and reducing agent) printing format is pictorially shown in figure 1.

In this cycle, after opening and washing the ink-jet printer cartridges, they were loaded separately with aqueous reducing agent and metal salt solutions. In each printing sequence, the cartridge was placed into the printer and the first pattern was printed on the substrate. Intermediate drying prevented subsequent contamination of the printer rollers in contact with the printing surface; the second cartridge loaded with metal salt was then used to ink jet exactly on top of the previously (reducing ink) printed areas. Metal formation occurred in situ as a consequence of the redox reaction between reducing agent and metal salt solutions leaving metallic layers composed of aggregated metal particles. The thickness of the deposited layer may be increased by repeating the ink-jet deposition process.
Non-reacted residues of reducing agent and metal salt can act as impurities between the deposited metallic particles and should be extracted in an after-treatment process. Gentle washing with water is a very simple method of extracting highly water-soluble impurities. Hot extraction was another method used in which a clean paper sheet was pressed on top of the dried deposited surface at high temperature (i.e. >100 °C) using a transfer printing press.

...
Silver ink-jet deposition
Silver has been used for centuries to reduce bacteria growth as well as being a key component in electronics. From a toxicological point of view and unlike other metals such as lead and mercury, silver is not toxic to humans and not known to cause cancer, reproductive or neurological damage, or other chronic adverse effects. Nor has normal day-to-day contact with solid silver coins, spoons or bowls been found to affect human health. This is because solid silver is almost completely biologically inert, and even if ingested, would pass through the human body without being absorbed into tissues. Silver nitrate is attractive as a potential metallic silver-bearing ink because of its high water solubility, stability and reasonable price. Ink-jet deposition of silver from silver nitrate solutions was most effective when using either ascorbic acid or hydroxylamine as reducing agent. Although hydroxylamine gave conductive prints, because of its irritant and possibly toxic nature, further ink-jet deposition experiments used ascorbic acid as the reducing substance.
A 40% (w/v) solution of ascorbic acid (pH 7–7.2) and a 67% (w/v) silver nitrate solution (pH 6.5) were used in the inkjet silver deposition trials. The reducing ink was printed first and after an intermediate partial drying at room temperature for 5 min the silver nitrate was overprinted. To extract the unreacted chemicals and their residues after the redox reaction, the silver deposited pattern was washed with water for 30 s and then hot pressed against a clean sheet of paper at 150'C for 20 s (hot extraction). Some yellow residual compounds, extracted from the printed pattern, are transferred to the clean paper during this hot pressing process. The magnified SEM images of the prints after the washing and hot extraction processes are shown in figure 7.
As is clearly visible (in the highly magnified image), the ink-jet deposition process is capable of producing nanosize silver particles on the substrate. A high concentration of the silver-bearing ink and fast reduction of silver ions to metallic silver are the main reasons for the formation of the particulate structure. Particle growth in a vertical direction has caused the formation of a very rough surface containing clusters of nanosize particles. The horizontal growth of each particle is usually limited by the growth of adjacent particles leading to a high degree of contact between the nanoparticles.
...
For each measurement, the ink-jet metal deposited sample was clamped in between two plates; one of these plates was fabricated with four copper probes which could contact the sample surface. Metal-deposited samples with a minimum length of 80 mm and of different print widths were measured; the actual length used in the resistance calculations was 10 mm, which was the distance between the two inner probes. To obtain accurate readings, the sample was secured between two plates using six screws which gave consistent and proper contact between their surface and the copper probes.
After clamping the sample between the plates, a constant current of 100 mA was supplied to the outer probes and the voltage between the inner probes was measured using a voltmeter. In this way, an average value of resistivity over the width of the film strip (i.e. effectively the integration of an infinite number of four-point probe measurements across the strip width) could be obtained.
...
Final calculated conductivity values are shown in table 1 for ink-jet deposited patterns on paper, ink-jet transparency film and cotton fabric. The minimum resistance values shown in the table are the result of dividing the measured voltage between the inner probes by the current applied to the outer probes of the four-contact device. The letters A and G in the deposition sequence column stand for ink-jet printing of ascorbic acid (A) and silver nitrate (G) inks, respectively.
Table 1. Maximum conductivity of the ink-jet silver patterns deposited on different substrates.
| Substrate | Deposition sequence |
Measured resistance (Ohms) |
Thickness (µm) |
Pattern width (mm) |
Pattern length (mm) |
Resistivity (×10-5 Ohm m) |
Conductivity (×10 5 S m-1) |
| Paper | AG | 3.57 | 3 | 9.5 | 10 | 1.02 | 0.983 |
| AGAG | 0.354 | 5 | 29 | 10 | 0.51 | 1.948 | |
| AAGG | 1.208 | 4 | 9 | 10 | 0.43 | 2.299 | |
| Ink-jet transparency film | AAG | 3.96 | 3 | 9 | 10 | 1.07 | 0.935 |
| AAGAG | 0.42 | 4 | 29 | 10 | 0.49 | 2.053 | |
| Cotton fabric | AAAGAG | 3 | 5 | 29.5 | 10 | 4.43 | 0.226 |
| AAAGAGAG | 0.871 | 6 | 26 | 10 | 1.36 | 0.736 |
These results are roughly 400 times worse than pure silver or copper, 65 times worse than steel, but more than twice as good as graphite and very usable for low current circuitry.
...
Printing PCBs, which are conductive tracks, can be simplified using the present deposition process as it is capable of depositing conductive tracks along with resistors and capacitors. A low conductivity track will work normally as a resistor and two parallel conductive lines can form a capacitor. The ink-jet technology described can deposit conductive tracks in the form of antennas for different devices such as mobile phones or radio frequency identification (RFID) tags. Some of the deposited silver patterns produced in the present research are shown in figure 9.

Some of the deposited silver patterns for different applications. (a) Mobile phone antennas on the transparency film. (b) Wiring boards printed on the transparency film. (c) Inductive coil printed on fabric. (d) RFID antenna with metallic joints printed on paper.
Also:
See also:
This paper introduces a low cost, fast and accessible technology to support the rapid prototyping of functional electronic devices. Central to this approach of 'instant inkjet circuits' is the ability to print highly conductive traces and patterns onto flexible substrates such as paper and plastic films cheaply and quickly. In addition to providing an alternative to breadboarding and conventional printed circuits, we demonstrate how this technique readily supports large area sensors and high frequency applications such as antennas. Unlike existing methods for printing conductive patterns, conductivity emerges within a few seconds without the need for special equipment.We demonstrate that this technique is feasible using commodity inkjet printers and commercially available ink, for an initial investment of around US$300. Having presented this exciting new technology, we explain the tools and techniques we have found useful for the first time. Our main research contribution is to characterize the performance of instant inkjet circuits and illustrate a range of possibilities that are enabled by way of several example applications which we have built. We believe that this technology will be of immediate appeal to researchers in the ubiquitous computing domain, since it supports the fabrication of a variety of functional electronic device prototypes.
"Reactive silver inks are synthesized by vortex mixing 1 g of silver acetate into 2.5 mL aqueous ammonium hydroxide at room temperature for 15 sec. Formic acid (0.2mL) is then titrated into the solution dropwise for 60 sec – vortex mixing after each drop. The solution changes in color from light orange to brown to grayish black indicating the rapid reduction of silver ions to large silver particles. The solution remains undisturbed for 12 h to allow the large particles to settle out yielding a clear supernatant that is decanted and filtered through a 200 nm syringe filter (Whatman Filters, Anotop 25). This clear solution, which contains 22wt% silver, serves as the reactive silver ink. "
"As ink dries, the labile ammonia ligands evaporate allowing the silver cations to be reduced by the formate anions as well as acetic acid produced in solution from the uncomplexed silver that was previously reduced. The combination of formate and acetate ions with acetic acid results in a solution that reduces to silver and silver acetate particles upon drying at room temperature. "
| file: /Techref/pcb/direct-inkjet-metal-traces.htm, 18KB, , updated: 2017/3/3 11:49, local time: 2025/5/17 02:18,
3.144.91.115:LOG IN
|
| ©2025 These pages are served without commercial sponsorship. (No popup ads, etc...).Bandwidth abuse increases hosting cost forcing sponsorship or shutdown. This server aggressively defends against automated copying for any reason including offline viewing, duplication, etc... Please respect this requirement and DO NOT RIP THIS SITE. Questions? <A HREF="http://sxlist.com/TECHREF/pcb/direct-inkjet-metal-traces.htm"> Extract from "Ink-jet fabrication of electronic components"</A> |
| Did you find what you needed? |
Welcome to sxlist.com!sales, advertizing, & kind contributors just like you! Please don't rip/copy (here's why Copies of the site on CD are available at minimal cost. |
Welcome to sxlist.com! |
.